Calculation of Activity at Constant Temperature
Purpose: Learn to calculate activities of components in a system
Module: PanPhaseDiagram
Thermodynamic Database: AlMgZn.tdb
Batch file: Example_#1.10.pbfx
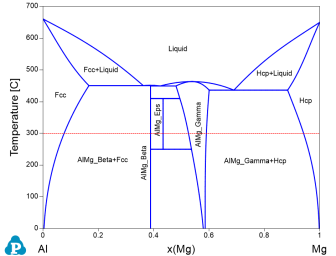
Figure 1 is the Al-Mg binary phase diagram. In this example, we learn to calculate the activity of Al and Mg as a function of composition, x(Mg), at 300°C (the red dash line).
Calculation Method 1, From menu bar “Property”:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database , and select Al and Mg two components;
-
Click “Property” on the menu bar and select “Thermodynamic Property”;
-
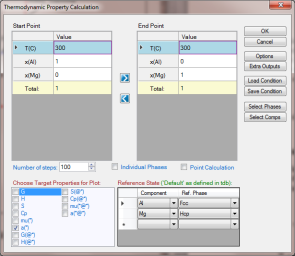
Set Calculation Condition as shown in Figure 2. The property selected is a(*), and the reference states are set as Fcc for Al and Hcp for Mg; (*) means the activity of every component (both Al and Mg in this case);
Calculation Method 2, From menu bar “PanPhaseDiagram”:
-
Load AlMgZn.tdb following the procedure in Pandat User's Guide: Load Database, and select Al and Mg two components;
-
Click “PanPhaseDiagram” on the menu bar and select “Line Calculation”;
-
Add the new table following the procedure in Pandat User's Guide: Icons for Table on Toolbar ;;
Post Calculation Operation:
-
Change graph appearance following the procedure in Pandat User's Guide: Property;
-
Add text and arrow on the plot following the procedure in Pandat User's Guide: Icons for Graph on Toolbar;
Information obtained from this calculation:
-
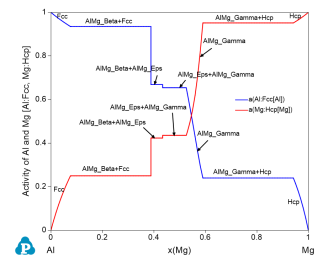
Figure 3 shows the activities of Al and Mg as a function of x(Mg) using Fcc Al and Hcp Mg as reference states;
-
Activity of Al is 1 at x(Al)=1 (x(Mg)=0) since it is pure Fcc Al at this temperature and Fcc is used as the reference state; Activity of Al decreases with the increase x(Mg) and becomes zero at x(Mg)=1;
-
Activity of Mg is 0 at x(Al)=1 (x(Mg)=0) and increases with the increase x(Mg) and becomes 1 at x(Mg)=1;
-
Activity of either component is constant in a two-phase field.